Specific Heat Capacity of Copper
Hence its derived SI unit is J kg1 K1. Specific heat capacity of Copper.
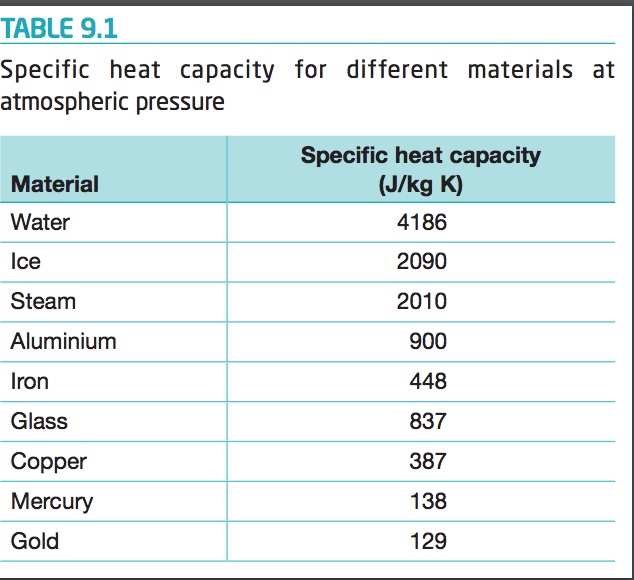
Solved Table 9 1 Specific Heat Capacity For Different Chegg Com
The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials and when applicable the molar heat capacity.

. Using the energy equation of Q ṁ x Cp x ΔT we can calculate the cooling capacity. Q c p m dt 1 where. The specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree CelsiusThe relationship between heat and temperature change is usually expressed in the form shown below where c.
C in Jgm K. Substance optional Custom Specific heat capacity c JkgK Advanced mode. By utilizing the high heat capacity of two-phase water cooling in a versatile copper package copper-water heat pipes are a popular component in a wide variety of thermal management solutions across broad range of industries.
This means that it takes 4200 J to raise the temperature of one kg of water by 1 C. A specific heat capacity calculator is functioned to deliver the outcomes along with standardized units. In an attempt to resolve these problems the standard lead grids were replaced with copper grids for the negative plates.
2 kg of carbon steel is heated from 20 o C to 100 o C. Specific heat capacity thermal expansion heat conduction thermal radiation and thermoelectric force are all aspects of thermal performance. The temperature change in a particular liquid heated by.
Units of Heat - BTU Calorie and Joule - The most common units of heat BTU - British Thermal Unit Calorie and Joule. Specific Heat Capacity of Metals Table Chart. The specific heat of water is partially responsible for the mild climate along Englands southwestern shore.
Specific Sub-Form Application System Standard Description. What is the specific heat capacity value of aluminum. LEADED RED BRASS CASTINGS SAND AND CENTRIFUGAL AS CAST.
Sulfur has a theoretical specific capacity and specific energy of 1672. Seamless copper tube for air conditioning and refrigeration field service. The heat capacity of a substance commonly abbreviated as thermal capacity capital C is a measure of the amount of heat needed to change the temperature of the substance by a specific amount.
A good website for this is peacesoftwarede the although we will need to convert the units to imperial so for that we will use Specific heat capacity and density of water. Heat capacity is measured in SI units and is referred to as the joules per kelvin JK unit. C in calgm K or Btulb F.
Table of Specific Heat Capacities. What is the specific heat capacity value of copper. 17000 modulus of rigidity ksi.
If specific heat is expressed per mole of atoms for these substances none of the constant-volume values exceed to any large extent the theoretical. The specific heat capacity of materials ranging from Water to Uranium has been listed below in alphabetical order. Q heat required kJ c p specific heat kJkg K kJkg C dt temperature difference K C Example - Heating Carbon Steel.
009 Modulus of Elasticity. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. Moreover the higher grid resistance causes increased heat generation in the cell and hence increased energy loss.
Gallium Specific heat capacity of Gallium. Thermal Conductivity Heat transfer characteristics of a solid material are measured by a property called the thermal conductivity k measured in Btuhr-ft-F. U-bend seamless copper and copper alloy heat exchanger and condenser tubes.
Heat Flux Heat Flux equation - The rate at which heat is transferred. Copper has a specific electrical conductivity σ 596 10 6 S m 1 and. 0092 modulus of elasticity in tension ksi.
Definition of Specific heat capacity revealed that it is the amount of heat required to increase the temperature of 1 kilogram of any substance by 1 kelvin. Temperature - Online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 C 32-700 F - SI and Imperial units. The energy required to heat a product can be calculated as.
U-bend seamless copper copper alloy heat. Specific heat capacity btu lb f at 68f. There are beaches as at Porthcressa Beach in Scilly where tropical plants grow.
Below this table is an image version for offline viewing. Water - Specific Heat vs. WROUGHT AND CAST COPPER ALLOYS.
Change of temperature ΔT C. Specific Heat Capacity Unit. An objects heat capacity describes the amount of heat required to change the temperature of that object by a certain amount.
5231 Specific heat capacity Specific heat capacity is the energy required to increase temperature of material of a certain mass by 1C in the unit of JkgK. While specific features will vary according to radiator application in general performance radiators have larger andor more tubes than factory radiators to increase coolant flow along with greater fin density to increase the surface area to which coolant and air are exposed which results in better heat transfer and a cooler running engine. Materials Specific Heat Capacity of Metals Table Chart.
Material JkgK BtulbmF JkgC kJkgK Aluminium 887 0212 887 0887 Asphalt 915 021854 915 0915 Bone 440 0105 440 044 Boron 1106 0264 1106 1106 Brass 920. Molar C Jmol K. Generally the most constant parameter is notably the volumetric heat capacity at least for solids which is around the value of 3 megajoule per cubic meter per kelvin.
We have set the worlds standard for commercial. Heat capacity is an extensive propertyThe corresponding intensive property is the specific heat capacity found by dividing the heat capacity of an object. Boyd Corporation has pioneered heat pipe development and solutions.
List of thermal conductivities Note that the especially high molar values as for paraffin gasoline water and ammonia result from calculating specific heats in terms of moles of molecules. This will give us a specifi heat capacity of 10007643BTUlbF and density of 62414lbFt3. The specific heat capacity.
The specific heat of aluminum is 897 Jkg K. Of water is 4200 joules per kilogram per degree Celsius JkgC. Copper Specific heat capacity of Copper.
Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius C. The specific heat of copper is 385 Jkg K. Specific heat capacity of Nichrome 80NI - 20 Cr 460548.
Specific Heat Capacity Btu lb F at 68F. Check out 36 similar. Specific heat is the amount of heat required to change the temperature of a substance by one degree generally C.
The SI unit of heat capacity is joule per kelvin JK. Thermal Diffusivity Table is the thermal conductivity divided by density and specific heat capacity at constant. Liquids absorb heat in different ways.
Nickel Specific heat capacity of Nickel.

What Is The Formula For Specific Heat Capacity A Plus Topper

Which Metal Heats Up Fastest Aluminum Copper Or Silver Chemdemos

Can Anyone Suggest A Material With The Highest Specific Heat Capacity Higher Than Water

Dublin Schools Lesson Specific Heat
0 Response to "Specific Heat Capacity of Copper"
Post a Comment